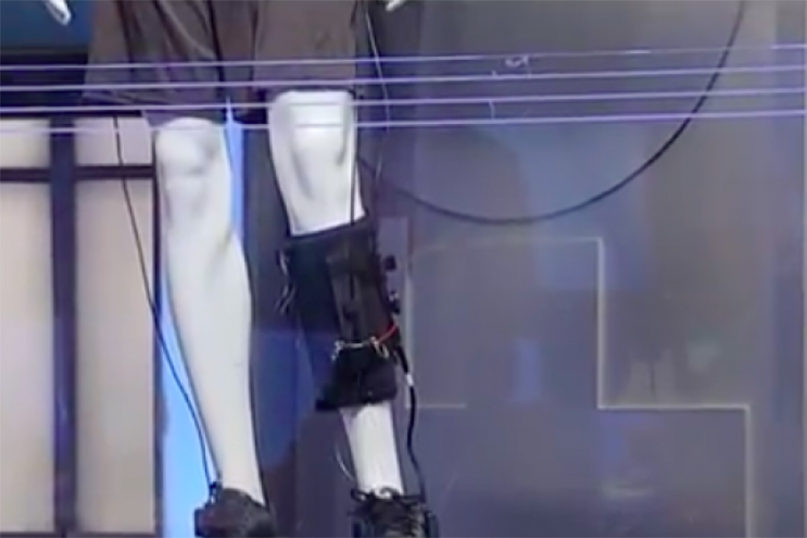
(JNS) The Food and Drug Administration has cleared a new product geared to help stroke survivors walk again. Israel-based ReWalk Robotics Ltd. announced on Tuesday, June 4 that its ReStore product will have a starting price in the United States of $28,900, though leasing options will be available. Last week, ReStore received the CE mark, allowing it to be sold in the European Union. ReWalk Robotics Ltd. is known for its exoskeleton that enables paraplegics to walk again. It received FDA clearance in 2014.
CAP: ReWalk Robotics Ltd.’s ReStore, an exoskeleton for stroke survivors. Credit: Screenshot.
SHARE








 Southern New England Jewish Ledger
Southern New England Jewish Ledger














